ISO 13485 Certification & Consulting Service in Kuwait
QUALIT
CERT
Qualitcert provides ISO 13485 certification and consulting services in Kuwait, catering to organizations involved in the medical device industry. ISO 13485 is an internationally recognized standard that sets out the requirements for a quality management system specific to the design, development, production, and servicing of medical devices. With a focus on ensuring regulatory compliance and product quality, Qualitcert’s expert consultants offer tailored solutions to help organizations navigate the complexities of the medical device industry. From establishing robust quality management systems to conducting internal audits and facilitating external audits for certification, Qualitcert supports clients throughout the certification process. By achieving ISO 13485 certification, organizations in Kuwait can enhance their credibility, demonstrate their commitment to quality and regulatory compliance, and gain a competitive edge in the global market for medical devices.
Please Reach Us Today

Please Reach Us Today


Approach and Methodology used to implement Management System Standard
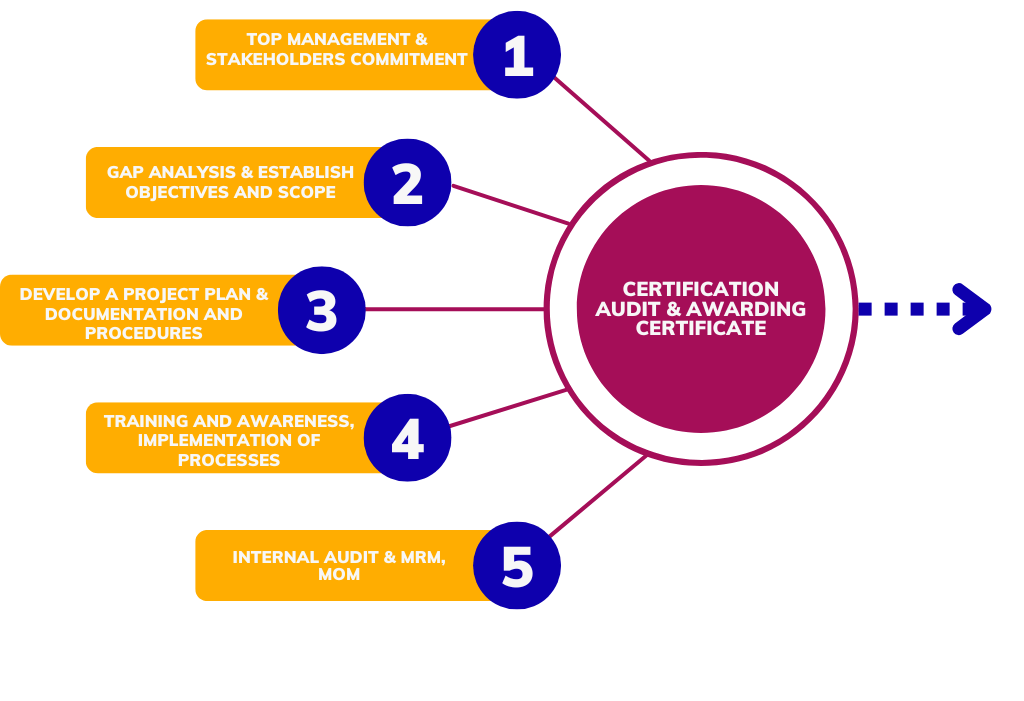
Implementing an ISO standards involves a structured methodology to ensure that the organization effectively meets the requirements of the chosen standard and achieves certification. Sometimes defined methodology may vary depending on factors such as the size of the organization, its industry, and the complexity of the ISO standard being implemented, the following steps provide a basic framework


OUR
Process
1, Determine the ISO Standard
2. Understand the Requirements
3. Training and Awareness
4. Implement the System
5. Internal Audit
6. Certification

Benefits of having ISO Certification
Enhanced Credibility and Reputation
Legal and Regulatory Compliance
Enhanced Customer Satisfaction
Access to Global Markets
Environmental Sustainability
Information Security
Our Achievements and Success
Our Clients





OUR
SERVICES
Our presence in Bangalore
Re-Design your system, create raving fan clients. Transform your business , Go Global
- ISO Certification in Peenya Bangalore
ISO Certification in Bommanahalli Bangalore
- ISO Certification in Whitefield Bangalore
- ISO Certification in Electronics City Bangalore
- ISO Certification in Bommasandra Bangalore
- ISO Certification in Rajajinagar Bangalore
- ISO Certification in Jigani Bangalore

