QUALIT
CERT
With a strong focus on helping medical device manufacturers meet critical international quality standards, Qualitcert offers ISO 13485 certification services in Lagos tailored to the unique needs of the healthcare and medical device industry. ISO 13485 is a globally recognized standard that governs quality management systems specifically for organizations involved in the design, production, installation, and servicing of medical devices. Through its expert consulting approach, Qualitcert assists businesses in Lagos in implementing effective quality controls, strengthening risk management practices, and ensuring full regulatory compliance across the entire product lifecycle. From initial system assessments and documentation support to internal audits and certification readiness, Qualitcert’s experienced team ensures a streamlined and efficient certification journey. Achieving ISO 13485 certification in Lagos not only enhances operational excellence and product safety but also reinforces brand reputation, builds trust among stakeholders, and opens access to both local and international medical device markets.
Get In Touch


Approach and Methodology used to implement Management System Standard
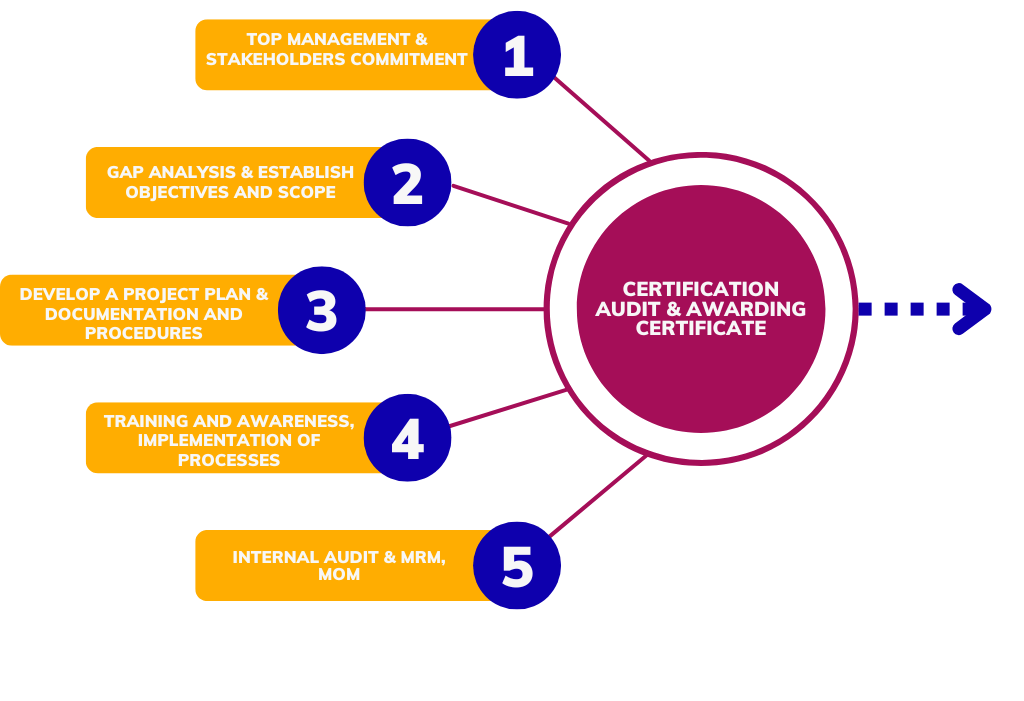
Implementing an ISO standards involves a structured methodology to ensure that the organization effectively meets the requirements of the chosen standard and achieves certification. Sometimes defined methodology may vary depending on factors such as the size of the organization, its industry, and the complexity of the ISO standard being implemented, the following steps provide a basic framework

OUR
Process
1, Determine the ISO Standard
2. Understand the Requirements
3. Training and Awareness
4. Implement the System
5. Internal Audit
6. Certification

Benefits of having ISO Certification
Enhanced Credibility and Reputation
Legal and Regulatory Compliance
Enhanced Customer Satisfaction
Access to Global Markets
Environmental Sustainability
Information Security
Our Achievements and Success
Our Clients






